Research
Circadian rhythms of behavior and physiology are found in organisms as diverse as bacteria and humans. Genetic screens in Drosophila led the way in identifying a set of “clock genes” that are required for 24 hour rhythms of activity in fruitflies. A very similar set of genes function in humans to control all kinds of rhythms ranging from sleep-wake cycles to metabolism. Justin gave a public talk in 2020 at NYU Abu Dhabi on how the insights from fundamental studies in Drosophila and mice are changing the way we think about medicine and human health.
The s-LNv circadian pacemaker neurons are the principal clock neurons in the Drosophila central brain. s-LNvs show dramatic 24 hour rhythms in their excitability and in the structure of their projections: They are maximally expanded around dawn along with their highest rates of firing; and retracted at dusk when they are much less excitable.
The predictability of this structural plasticity combined with the power of Drosophila genetics makes s-LNvs ideal “model neurons” to study fundamental mechanisms of neuronal plasticity that we hope will be generalizable to other neurons. We are studying the plasticity of these clock neurons in 3 main areas:
1. Transcriptional regulation of neuronal plasticity
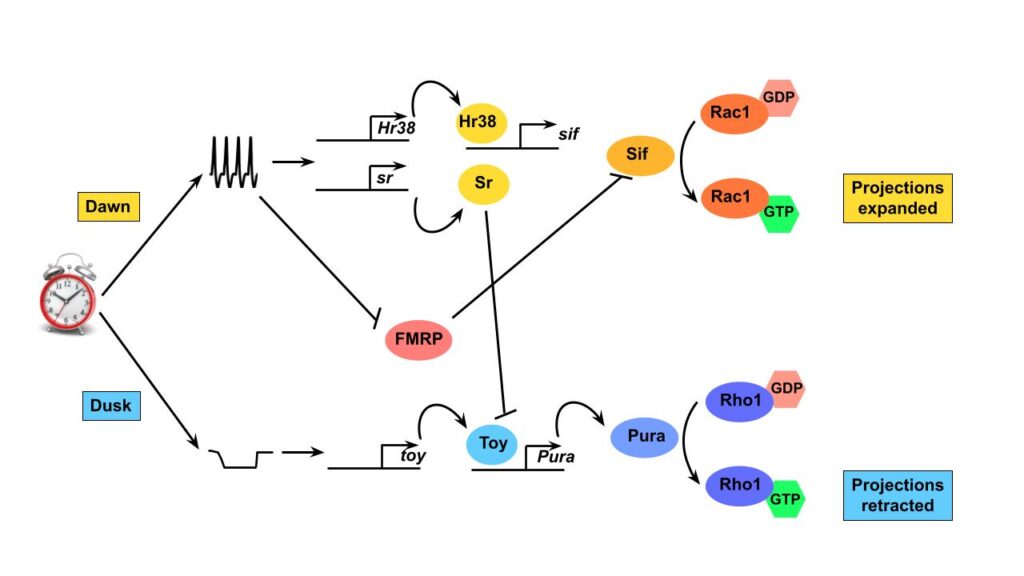
Many mammalian neurons rapidly activate activity-regulated genes (also known as immediate early genes) in response to neuronal activity. These activity-regulated genes then go on to participate either directly or indirectly in synaptic plasticity. In a recent preprint, we showed that Drosophila s-LNs use activity-regulated genes to expand their projections at dawn. Conversely, we have found that s-LNvs use a more novel inactivity-regulated gene expression program to retract their projections at dusk in response to low levels of neuronal activity. We want to understand the signaling pathway that activates inactivity-regulated genes and test whether this is a general property of neurons.
2. Post-transcriptional regulation of neuronal plasticity
RNA-binding proteins are key regulators of neuronal plasticity – for example, the Fragile X mRNA binding protein FMRP, which is mutated in Fragile X Syndrome and is also the largest single gene cause of autism spectrum disorder. Although Fragile X and autism spectrum disorder are considered neuro-developmental disorders, we recently found that FMRP functions in adult fully developed s-LNvs to regulate their structural plasticity bi-directionally: too much FMRP at dawn stops s-LNvs from expanding normally, while not enough FMRP at dusk stops s-LNvs from retracting. We also used a clever genomic method from the Rosbash Lab (TRIBE-seq) to successfully identify FMRP target genes in s-LNvs, even though there are only 8 s-LNvs per brain.
3. Beyond the s-LNvs – making and breaking neuronal connections:
How do s-LNvs know which connections to make and break during plasticity? To begin to address this question, we adapted the innovative Trans-tango method from the Barnea Lab to identify s-LNv connections using single-cell RNA sequencing – and we call this combination of methods Tango-seq. Future work is aimed at identifying the molecular code that directs s-LNvs to the appropriate partner neurons. We are also testing the role of glia in s-LNv plasticity.